🥬 The Magic of Red Cabbage: A Natural pH Indicator
Ever wondered how scientists test whether something is an acid or a base? You don’t need a lab to find out—you just need red cabbage! This vibrant vegetable contains a natural pH indicator that changes color depending on the substance it touches. In this experiment, we’ll use red cabbage to reveal the hidden chemistry of everyday items.
Materials
½ head of red cabbage
Hot water
Blender or pot & strainer
Clear cups or glasses
Substances to test: vinegar, baking soda solution, lemon juice, soap water, soda, milk, etc.
Steps
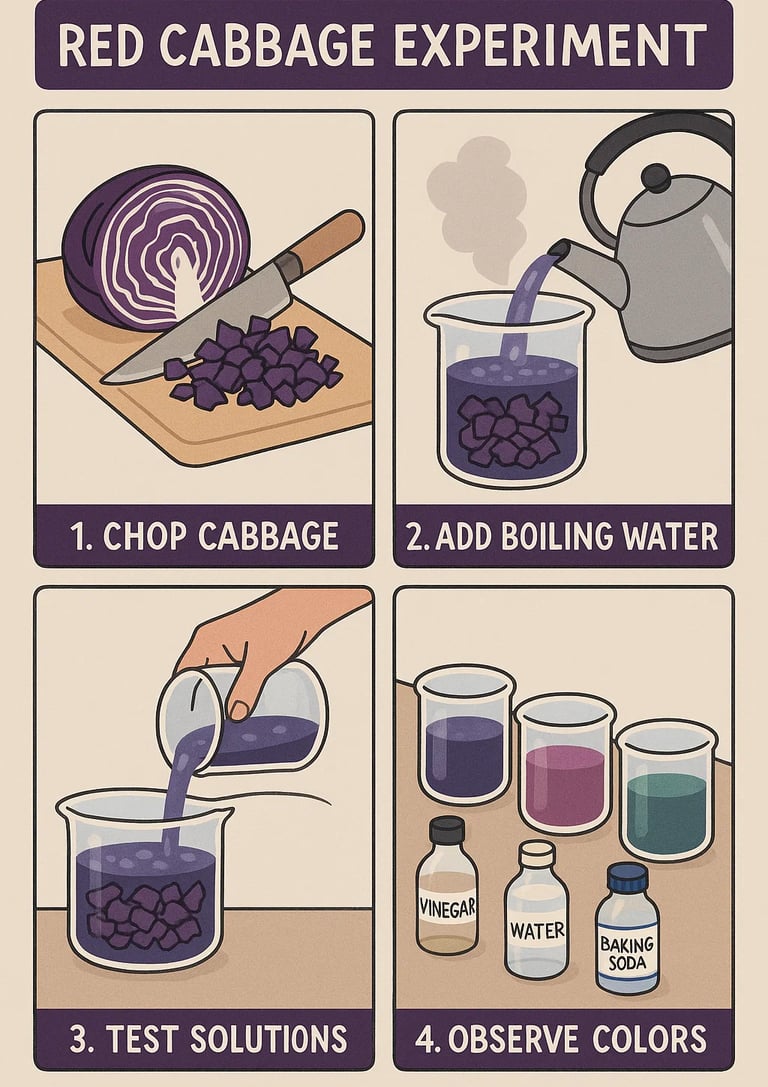
Make the cabbage indicator: Chop the cabbage and blend it with hot water. Let it sit for 10 minutes, then strain to keep the purple liquid—this is your pH indicator.
Pour into cups: Fill small amounts of the cabbage juice into separate clear cups.
Add test substances: Add a small amount of each household liquid to its own cup of cabbage juice and observe the color change.
Record results: Make a color chart from acidic (reddish) to basic (greenish/yellow).
What’s Happening?
Red cabbage contains anthocyanins, pigments that change color depending on pH.Acids (like lemon juice or vinegar) turn the solution pink/red.
Bases (like baking soda or soap) turn it green or yellow.
Neutral substances may leave it purple.
Applications
Red cabbage indicators are a great teaching tool in classrooms.
Scientists use synthetic indicators like litmus or phenolphthalein for precise measurements.
Natural indicators are eco-friendly alternatives for introductory chemistry.
Safety Tips
Handle hot water carefully when preparing the cabbage extract.
Some substances may cause skin irritation—use gloves if needed.
Don’t taste-test the mixtures, even if they’re made from kitchen items.
